ORANGE BIOMED TO PRESENT BREAKTHROUGH MICROFLUIDIC TEST FOR LAB-ACCURATE HbA1c IN MINUTES AT ADLM 2025
Novel approach analyzes thousands of red blood cells from a small sample, showcasing the long-term potential of microfluidics for testing across conditions
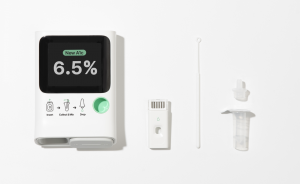
Using advanced microfluidic technology, our platform analyzes thousands of individual red blood cells from a single drop of blood within just five minutes.”
CHICAGO, IL, UNITED STATES, June 26, 2025 /EINPresswire.com/ -- Orange Biomed—the inventor of the world’s first pocket-sized, microfluidic-based A1C analysis device, dedicated to improving access and accuracy in chronic condition care—has been selected to present a poster at the ADLM 2025 Clinical Lab Expo. Orange Biomed will detail its new, novel method of testing HbA1c, which utilizes microfluidic technology to generate lab-accurate results within minutes from a minimal blood sample. Presentations will take place from 1:30 PM to 2:30 PM on July 29 in Chicago, IL. Orange Biomed recently launched M.A.P. Your Health—a nationwide campaign designed to inspire and empower community members to monitor their risk factors and prevent chronic disease. Educating key ecosystem players across all areas of lab medicine on the potential impact of microfluidics-based testing at ADLM supports this mission, ahead of the M.A.P. Your Health Chicago: Community Resource Fair and Public Health Forum event this August. — Yeaseul Park, CEO and Co-Founder of Orange Biomed
“Using advanced microfluidic technology, our platform analyzes thousands of individual red blood cells from a single drop of blood within just five minutes; significantly reducing the sample size required and enabling faster, easier testing,” said Yeaseul Park, CEO and Co-Founder of Orange Biomed. “This isn’t simply a faster version of traditional testing; it’s a fundamentally new approach. We enable more precise HbA1c measurements, which are critical for diabetes care. Many remain under-monitored due to barriers to lab testing. Our solution brings high-quality monitoring into homes, clinics, and rural settings. Aligned with ADLM’s theme of ‘Bring the Wonder,’ we will showcase the long-term potential of microfluidics beyond diabetes to improve chronic condition care diagnostics and management in the U.S.”
Orange Biomed’s poster, A-386 titled “A novel microfluidic-based HbA1c testing method by analyzing the stiffness of red blood cells,” will be presented in the poster hall and on display from 9:30 AM – 5:00 PM on July 29. The company aims to educate clinical labs, diagnostic companies, OEMs, and other healthcare leaders on the long-term potential of microfluidics in optimizing patient care across a range of conditions. Dr. Unghyeon Ko, Co-Founder and President of Orange Biomed, who is spearheading R&D efforts, added, “The potential of microfluidics extends far beyond diabetes. This technology enables personalized medicine, where treatment is based on each person’s unique biological profile.”
He furthered, “As microfluidic systems evolve, we envision a future where more innovators apply this technology to medical devices to form multiplexed diagnostics that integrate genomics, proteomics, and metabolomics into a single, compact platform. Imagine a future where a device on your bathroom shelf can track immune status, detect early signs of cancer, or assess medication response, all from a few drops of blood. That future is closer than we think, and microfluidics is the gateway.”
About Orange Biomed
With U.S. headquarters in Seattle, WA, Orange Biomed was launched in 2021 by Duke University alumnus Dr. Unghyeon Ko and Yeaseul Park to solve unmet diabetes-focused healthcare needs. The healthcare startup innovates cutting-edge technology for diabetes management, including OBM rapid A1c.
The global impact of OBM rapid A1c was recognized in 2024 when the Korean Hospital Association honored it with the prestigious KHF Innovation Award for its revolutionary application of microfluidic technology conducting single-cell analysis.
In 2025 and beyond, Orange Biomed is focused on bringing OBM rapid A1c to U.S. patient-oriented healthcare. The company is preparing a 510(k) submission to the FDA for potential over-the-counter (OTC) clearance of its product, followed by a point-of-care (POC) application submission, further expanding access to innovative diabetes care solutions and more.
Learn more: https://www.orangebiomed.com
About the “M.A.P. Your Health” Campaign
Orange Biomed, in collaboration with local healthcare leaders, is the flagship sponsor of “M.A.P. Your Health”—a nationwide campaign dedicated to empowering communities to monitor and manage their risk factors for chronic diseases.
M.A.P. stands for Monitor A1C regularly, Adapt your treatment plan, and Prevent the risk of long-term complications.
Through this campaign, Orange Biomed aims to close gaps in care and prevent long-term complications by raising awareness and encouraging individuals to take proactive steps to manage their health, regularly monitor their A1C, and use critical tools and resources to safeguard their long-term well-being.
Take action today: https://map.orangebiomed.com
Shannon Lindahl
Orange Biomed
+1 781-733-6973
email us here
Revolutionary New Method of Testing HbA1c
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.